World’s First Lung Cancer Vaccine BNT116 Enters Global Trials, Signalling a New Era in Oncology
A major medical breakthrough has been announced: BNT116, the world’s first lung cancer vaccine, has officially entered clinical trials across seven countries. This ambitious project is underway at research centers in the United States, United Kingdom, Germany, Hungary, Poland, Spain, and Turkey.
BNT116 was developed by BioNTech, the biotech company behind one of the pioneering COVID-19 vaccines. The vaccine specifically targets non-small cell lung cancer (NSCLC), the most common type of lung cancer globally. Utilizing the same proven mRNA technology platform, BNT116 works by training the body’s immune system to precisely recognize and attack cancer cells, potentially preventing recurrence and significantly improving long-term patient survival.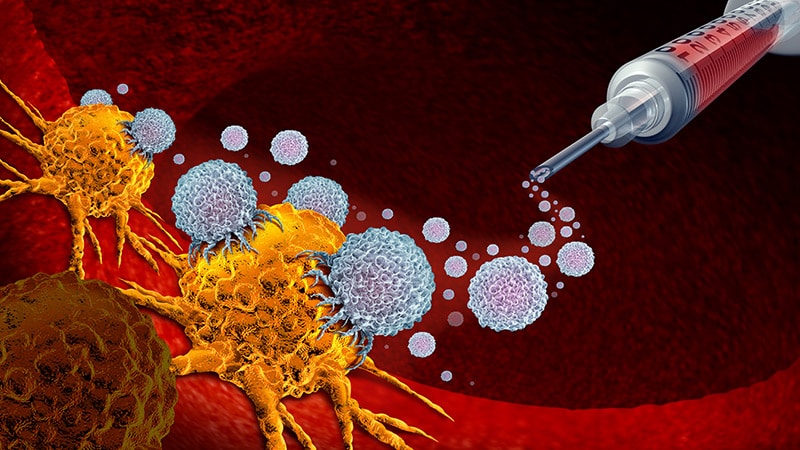
The ongoing Phase 1 study involves approximately 130 patients across 34 clinical sites, primarily focusing on assessing safety, tolerability, and immune response. Researchers are immensely hopeful that this vaccine could become a powerful new weapon in the fight against a disease that tragically claims over 1.8 million lives each year.
If successful, BNT116 is set to mark a major shift in the cancer treatment paradigm—moving away from reactive chemotherapy toward proactive immunotherapy. This would usher in an entirely new era of personalized, precision medicine.